Michele Rehbein is a double masters student currently attending Western Illinois University in the programs of Biology and Health Sciences, specifically focusing on microbiology and public health. She has been conducting research in the immunology-virology lab at WIU on West Nile virus. She plans to get her PhD after she has graduated from WIU, but in the meantime has interests in continuing research and gaining experience. This blog was created as a class project through a medical mycology course she is currently in.
Madurella
General Description:
Madurella
is a filamentous fungus found in the soil. It is described as dark-walled or
phaeiod, called dematiaceous, which means they are brown-pigmented (9). It is
commonly found in tropical and subtropical areas such as Africa, India, and
South America. Madurella is a
pathogenic fungus that causes infections in humans.
 |
| Grains of M. grisea.
www.mycology.adelaide.edu.au
|
 |
| M. mycetomatis
www.mycology.adelaide.edu.au
|
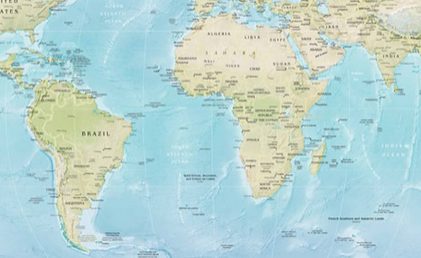
Distribution:
Madurella
mycetomatis causes eumycetoma in individuals living in arid climate zones
of east and west regions of Africa, specifically Somalia, Djibouti, Sudan,
Nigeria, Mali, and Senegal, and it occasionally is found in the Middle East,
and in southern India (12). M. grisea
is a common etiologic agent found in South America (12). Other cases have been
reported in temperate regions, such as the Appalachians in the United States,
but is rarely found in Europe (8). Although the highest incidence of eumycetoma
is found in Sudan from June through October when the hot, rainy seasons occur,
the disease can be frequently imported into Western countries and the United
Kingdom due to an increase of immigrants and tourists (5, 8).
Habitat:
 |
| nilscordes.com |
Risk factors:
Males, ages 20 to 45 years old, are
more susceptible than females, with approximately a ratio of 4:1 (12). This
higher number of infection rates in men is very likely related to their occupations.
Men tend to hold more jobs outdoors such as farmers, herders, and agriculture. Individuals
who work outdoors in rural areas without proper footwear or proper foot
protection can get this fungal infection from traumatic implantation. Some
areas also do not have the proper medical or health care services and resources
to properly treat the infection even if an early diagnosis is made (2). In some
instances, some individuals cannot afford treatment either. Commonly seen is
that most patients who are admitted for this type of infection often lack any
knowledge or education of it (2).
Eumycetoma has been associated with
patients who have generally normal health and is not associated with those who
are immunosuppressed. However in some cases, a decreased activity level
resulting from having the disease can actually lead to economic depression
within communities (12). The economy can be hurt due to the amount of diseased
individuals not able to attend their jobs, therefore reducing workforce
efficiency. Between the years of 1979 and 1985, there was a study of 27
patients in the United Kingdom and 12 (44%) of these patients had diabetes
mellitus that also had infection of eumycetoma (12).
Veterinary forms:
Madurella
spp. have been found to cause disease in dogs. It can cause dermal or
visceral focal phaeohyphomycosis (14). Dogs that are infected present symptoms
of focal granulomas with viscera as well as fever and malaise, and there may
also be intracranial infection (14). Eumycetoma has also been found in horses,
cattle, occasionally cats, and sometimes exotic animals, such as squirrel
monkeys and parrots (12).
Transmission:
The main route of transmission for
this fungus is intracutaneous (within the skin) inoculation (12). Infection can
follow traumatic implantation of fungal spores or hyphal fragments that are
present in the soil or on plant material (5). Eumycetoma is not transmissible
between humans, animals to humans, or humans to animals (12).
Other fungi that cause dark grain
mycetoma is Pyrenochaeta romeroi, Pseudallescheria boydii, and Scedosporium apiospermum (12). However,
there has not been much data existing that understands the genetic relationship
between these organisms and the possibility of other fungi that might provoke
mycetoma (5).
 |
| Madura foot.
www.lucianoschiazza.it
|
Histopathology/Pathogenesis:
M.
mycetomatis is the most common causative agent of
eumycetoma worldwide (10). Mycetoma is a chronic, subcutaneous granulomatous,
progressive and destructive inflammatory disease cause by true fungi (10). A
mycetoma caused by a true fungus is classified as eumycetoma (10). Pathogenesis
of mycetoma is not completely understood or known (2). It has also been an
ignored disease for many years by international health organizations, until
recently when the World Health Organization (WHO) recognized it as a neglected
tropical disease (1).
Infections of eumycetoma tend to be
slow and progress over time (8). These infections could potentially spread to
the skin and deep structures such as bone. This could lead to deformity and
destruction, and loss of function/disability. Sometimes an infection can become
fatal. The most commonly affected body
part of eumycetoma is the foot, with approximately 70% of cases observed (8).
However other exposed body parts can get this disease. Other body parts seen
with infection are the hand, leg, knee, arm, thigh, and perineum (8).
Craniofacial mycetoma is rare, and there have also been infections seen in the
paranasal sinuses, mandible, intraspinal, bladder, brain, and lung (8).
 |
| Periodic acid-Schiff stained section showing grains with broad hyphae.
Arenas et al., 2009.
|
 |
| Grains from infected specimen.
Arenas et al., 2009.
|
Pus, exudates, or biopsy material with grains are the primary indicators of eumycetoma (12). Taking into account the size, shape, color, and consistency of the grains also help with identification of which fungus may be the cause, such as Madurella. The grains caused by hyaline molds with be white or yellow, and grains caused by melanized fungi will be black (12). KOH prep can be used and staining with lactophenol cotton blue or lactofuchsin can be used. Eumycetoma grains for culture can be washed with sterile water or saline containing antibacterial antibiotics and they can be crushed and cultured in duplicate (12). Media that is suggested is SDA and brain heart infusion (BHI) with choloramphenicol, or penicillin and streptomycin, but cyclohexamide is not used as it may inhibit some causative fungi (12). The incubation temperatures should be between 25-30°C, and also keeping in mind some species are slow growing fungi, the cultures should be kept for 6-8 weeks (12).
Therapy:
Eumycetoma is difficult to treat and
a major challenge. Therapy failure is common and there is also a high rate of
the fungal disease to recur (1). Surgical treatment may sometimes be the only
option if the lesion is very well defined (2). If there are very advanced
lesions present, treatment with antifungal agents may be very ineffective,
especially when it has progressed to the point where the bone is involved (12).
Surgery in combination with azole treatment in recommended for smaller
eumycetomas on extremities (2). Some reports suggest that voriconazole is a
good azole to use for treatment for black grain mycetoma, itraconazole also
helps to stabilize lesions prior to surgery (2). There have been reports
showing that M. mycetomatis is the
most susceptible to the azole class of antifungal agents with ketoconazole and
itraconazole being the most frequently used drugs for treatment of it (1). A
study done by Ahmed et al. 2014 also showed M.
mycetomatis to be susceptible to ravuconazole.
Early diagnosis is a must and intervention
is needed immediately with antimycotics potentially accompanied by surgery to
eliminate the need for more serious surgical treatment and amputation (2).
Clinical
Case Examples:
1.
A 39 year old man living in Mali presented signs of chronic swelling with
sinuses that showed draining purulent fluid in his left ankle (12). He decided
to go to a clinic for treatment in December of 2000 which was located in Paris,
France. The man had past history of surgical removal of an abscess near the
current affected ankle with black grains found. He had been treated with
trimethoprim-sulfamethoxazole, but had little to no improvement. A lytic lesion
was found from radiography in his distal fibula and one week after the man had
sought treatment he was taken into surgery for massive excision of the soft
tissue and the infected bone (12). Specimens taken from the surgery showed
numerous black grains. The black grains were washed in sterile water and
cultured on SDA and chocolate agar. The plates were incubated at 30°C and 37°C
and after 4-5 days a slow growing mold appeared. After 8 weeks of incubation no
conidia were seen, and no identification could be done by observing morphology.
PCR and sequencing of rDNA using ITS1 and ITS4 were done and Madurella mycetomatis was identified. There
was additional surgery completed and the man was also given treatment of
amoxicillin and itraconazole twice a day for 20 weeks (12). After a 2 month
follow up, there was no recurrence of the fungal infection seen, however two
years following the admittance of this man, his wound was still found to be
draining (12).
2.
A 21 year old farmer had a four year
history of a nodular plaque involving the arch of the plantar surface of the
right (2). The nodular plaque had developed after trauma to the foot while the
farmer was working in the fields. The patient had been surgically treated
within their community but scarring had developed, and recurrence appeared after
6 months from the date of the surgery. The patient had a lesion which had
bloody drainage with serous exudate and black charcoal-like granules or grains
(2). Using Lugol’s iodine, the grains were observed, and a biopsy was taken
from the deep dermis near one of the fistulas (2). The biopsy showed filaments
at the periphery with suppurative granuloma containing neutrophils, fibrous
stroma, and Langerhans-type giant cells (2). Gomori-Grocott and periodic
acid-Schiff (PAS) stains were used and cultures of the grains of SDA and
Mycosel agar were positive for M.
mycetomatis (2). By using X-ray and computed topography scan it was shown
that only soft tissue not bone was affected by the infection (2). The patient
was treated with itraconazole for 300 mg daily for six months but only a mild
decrease in the inflammation occurred. Surgical removal was needed since there
was only partial closing of the fistulae. Topical negative pressure (TNP)
therapy was given to the patient to stimulate granulation tissue formation
followed by an autologous skin grafting (2). For an additional 3 months, the
patient was given itraconazole and by 18 months the patients was disease free.
Related
Links:
References:
1.
Ahmed, S.A., de Hoog, G.S., Duncanson, F., Fahal, A.H., Kloezen, W., van de
Sande, W.W., & Zijlstra, E.E. (2014). Madurella mycetomatis is highly
susceptible to ravuconazole. PLoS
Neglected Tropical Diseases, 8(6). doi: 10.1371/journal.pntd.0002942.
2.
Arenas, R., Chavez-Lopez, D., Dominguez-Cherit, J., Estrada-Castanon, R.,
Estrada-Chavez, G.E., Fernandez, R., Hay, R., & Vega-Memije, M. (2009).
Eumycotic mycetoma caused by Madurella mycetomatis successfully treated with
antifungals, surgery, and topical negative pressure therapy. International Journal of Dermatology, 48,
401-403.
3.
Belkum, A., Fahal, A., van de Sande, W.W. (2013). Mycetoma caused by Madurella
mycetomatis: A completely neglected medico-social dilemma. In: Hot topics in infection and immunity in
children IX. Springer New York Dordrecht Heidelberg London (p. 179-189).
4.
Belkum, A., Fahal, A., Riley, T.V., van de Sande, W.W., & Verbrugh, H.
(2007). In vitro susceptibility of Madurella mycetomatis, prime agent of Madura
foot, to tea tree oil and artemisinin. Journal
of Antimicrobial Chemotherapy, 59. doi: 10.1093/jac/dkl526.
5.
Borman, A.M., Johnson, E.M., Linton, C.J., & Miles, S. (2008). Molecular
identification of pathogenic fungi. Journal
of Antimicrobial Chemotherapy, 61. doi: 10.1093/jac/dkm425.
6.
Charlier, C., Consigny, P.H., Lebeaux, D., Lecuit, M. & Lortholary, O.
(2012). Fungal infections in immunocompromised travelers. Clinical Infectious Diseases, 56(6), 861-869. doi: 10.1093/cid/cis935.
7.
Dankert, J., Guchelaar, H.J., & Vermes, A. (2000). Flucytosine: A review of
its pharmacology, clinical indications, pharmacokinetics, toxicity and drug
interactions. Journal of Antimicrobial
Chemotherapy, 46, 171-179.
8.
Deng, J., Hao, F., Yan, J., Zhong, B., & Zhou, C. (2009). Phenotypic and
molecular characterization of Madurella pseudomycetomatis sp. nov., a novel
opportunistic fungus possibly causing black-grain mycetoma. Journal of Clinical Microbiology, 48(1),
251-157. doi: 10.1128/JCM.00018-09.
9.
Doctor Fungus. (n.d.) Madurella spp. Retrieved April 7, 2015 from http://www.doctorfungus.org/thefungi/Madurella.php.
10.
Fahal, A., Ibrahim, A.I., Hassan, A.M., & van de Sande, W.W. (2013). A
histopathological exploration of the Madurella mycetomatis grain. PLoS ONE, 8(3). doi: 10.1371/journal.pone.0057774.
11.
Liu, D. (2011). Molecular detection of
human fungal pathogens. Boca Raton, FL., Taylor & Francis Group.
12.
Lyon, M.G., Reiss, E., & Shadomy, J.H. (2012). Eumycetoma (Madura foot,
maduramycosis). In: Fundamental medical
mycology. Hoboken, New Jersey: John Wiley & Sons, Inc. (p. 513-522).
13.
Madurella mycetomi. (2015). Retrieved April 12, 2015 from http://www.mycobank.org/name/Madurella%20mycetomi.
14.
Madurella spp. (2012). Retrieved April 14, 2015 from http://www.vetbook.org/wiki/dog/index.php/Madurella_spp.
15.
Mycology
Online. (2015). Madurella spp. Retrieved April 7, 2015 from
http://www.mycology.adelaide.edu.au/Fungal_Descriptions/Hyphomycetes_%28hyaline%29/Madurella/.